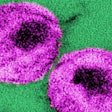
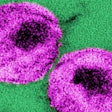
The U.S. Food and Drug Administration (FDA) has approved Hologic's diagnostic claim for its HIV-1 viral load monitoring assay, the Aptima HIV-1 Quant Dx assay.
The Aptima HIV-1 Quant Dx is now the first dual-claim assay for both diagnosis and viral load monitoring in the U.S., Hologic said. First approved in late 2016 for viral load monitoring, the assay is a molecular diagnostic test that runs on the automated, sample-to-result Panther system. It uses a dual target approach against highly conserved regions in the HIV genome designed to deliver results across HIV-1 groups and subtypes, Hologic said.