Artificial intelligence (AI) healthcare company Avenda Health on Wednesday announced that its cancer management platform, iQuest, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
Avenda recently raised $10M in Series B funding to accelerate the use of iQuest and continue clinical evidence development.
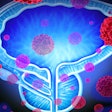
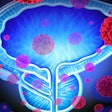
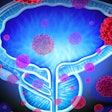
iQuest uses deep-learning algorithms with existing patient-specific diagnostic information to create a tailored map of the cancer's location within the prostate. The platform provides physicians with a 3D visualization, enabling treatment decisions that consider the extent of the cancer rather than treating the whole organ.
The current approach is to treat the entire prostate, as current MRI technology cannot identify the full extent of the tumor and cancer growth within the prostate. This approach results in nearly 50% of patients losing their sexual or urinary function. An approach that identifies the precise area affected by cancer, as iQuest does, offers a better understanding of the extent of the disease and aids in treatment planning that can preserve the patient’s quality of life while minimizing the risk of leaving any remaining cancer.
iQuest has been validated in multiple clinical trials. In one study, the use of iQuest enabled urologists to improve their sensitivity of identifying tumor extent from 37% to 97%. Furthermore, the urologists changed their treatment recommendation in 27% of cases, primarily toward more localized treatment.