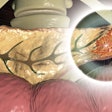
Bluestar Genomics on Wednesday announced both its renaming as ClearNote Health and the U.S. and international commercialization of its first test for the early detection of pancreatic cancer in high-risk patients.
The newly rebranded ClearNote Health also announced an international distribution agreement with LifeStrands Genomics, part of the Genomics and Life Sciences division of Singapore-based Pathology Asia, a diagnostic service provider in southeast Asia. LifeStrands Genomics will distribute ClearNote Health’s pancreatic cancer early-detection test in Asia, starting with Singapore and Malaysia.
The test is a DNA-based blood test which uses the 5-hydroxymethylcytosine (5hmC) biomarker along with other genomic features to determine if a patient has an abnormal DNA signal associated with pancreatic cancer. It is already available in the U.S. through the ClearNote Health physician experience program. All of the tests are performed in ClearNote Health’s CAP-accredited, CLIA-certified laboratory in San Diego.
ClearNote Health shared results from its validation study of the test at the American Pancreatic Association annual meeting in November.