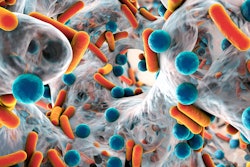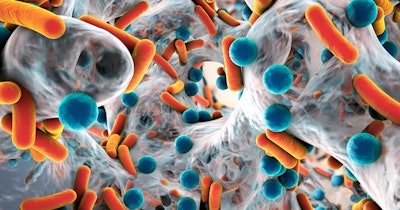
Researchers are hailing progress toward more rapid detection of antimicrobial resistance (AMR), with a susceptibility test returning results in 30 minutes.
University of Oxford researchers say that developing the system, which combines artificial intelligence (AI) with fluorescence microscopy, could hamper the rise of AMR and lead to precision treatments for the sickest patients.
The team trained deep-learning models to analyze bacterial cell images and detect structural changes that may occur in cells when they are treated with antibiotics. The method was shown to be effective across multiple antibiotics, achieving at least 80% accuracy on a per-cell basis, according to findings published in Communications Biology.
The Oxford University study demonstrates a “proof-of-principle approach to rapid single-cell antibiotic susceptibility profiling,” which in principle could address some shortcomings of existing assays. The deep-learning models were able to detect antibiotic resistance reliably and at least 10 times faster than established “gold-standard” clinical methods, say the authors. Current testing methods which rely on growing bacterial colonies in the presence of antibiotics are slow, often requiring several days to show how resistant bacteria are to a range of antibiotics.
The approach was deployed on cell populations from six Escherichia coli strains obtained from human bloodstream infections with varying degrees of ciprofloxacin resistance and treated with a range of ciprofloxacin concentrations. The study found that “single-cell phenotyping has the potential to provide equivalent information to growth-based antimicrobial susceptibility testing (AST) assays, but in as little as 30 min.”
The researchers hope that the model could be used to identify whether cells in clinical samples are resistant to a wide variety of antibiotics in the future.
“Antibiotics that stop the growth of bacterial cells also change how cells look under a microscope, and affect cellular structures such as the bacterial chromosome,” said study co-author Achillefs Kapanidis, professor of biological physics and director of the Oxford Martin Programme on Antimicrobial Resistance Testing.
“Our AI-based approach detects such changes reliably and rapidly. Equally, if a cell is resistant, the changes we selected are absent, and this forms the basis for detecting antibiotic resistance,” Kapanidis added.
The researchers say that further work is required to validate their approach with more species and antibiotics. They hope the method may facilitate targeted antibiotic treatments, helping to decrease treatment times, minimize side effects, and ultimately slow down the rise of AMR.
The authors say the technique could also serve as a “richer potential source of clinical information” compared to current assays.
“Currently established ASTs operating at the colony level only offer aggregate, sample-wide information measured through secondary markers correlated with resistance, such as culture growth or genomic information. This might miss relevant mixed bacterial populations or important heteroresistance phenotypes which could be picked up by evaluating individual cellular susceptibility phenotypes using our method,” they write.
Researchers from the Oxford University Hospitals National Health Service Foundation Trust’s Department of Microbiology and Infectious Diseases also contributed to the study.
The Oxford Martin School has produced a guide to the AMR testing process that is available on its website.