The College of American Pathologists (CAP) on September 9 released new draft recommendations on biomarkers for diffuse gliomas, the most common group of primary brain tumors. The move recognizes the growing importance of molecular testing in tumor classification.
CAP has drafted 13 recommendations related to testing for diffuse gliomas, with the guideline open for public comment through September 30. The draft recommendations include strong endorsements for IDH mutational testing in all diffuse gliomas and for MGMT promoter methylation testing in all cases of glioblastoma. CAP also included guidance on the management of patients with a 1p/19q codeletion.
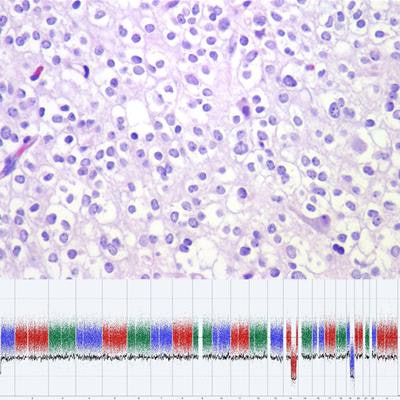
 Image courtesy of Dr. Daniel J. Brat, PhD.
Image courtesy of Dr. Daniel J. Brat, PhD.An estimated 15,000 people are diagnosed with diffuse gliomas every year in the U.S. The classification of this family of tumors has evolved to become much more complex, with many different known molecular subtypes, CAP noted.
"We thought that diagnostic testing for this family of tumors had matured enough and had evidence solid enough that we could provide guidance to the practicing pathologist out there who maybe does not see that many brain tumors in their practice," guideline development lead Dr. Daniel J. Brat, PhD, commented in an interview.
Brain cancer diagnosis evolves
Tumors have traditionally been classified based on microscopic analysis, with grading of structural characteristics per World Health Organization classifications. But pathologists don't just make a diagnosis based on what they see under the microscope -- nowadays, they incorporate genetic findings into a final diagnosis, and the field is continually evolving.
"We've come into so much of a new era in the past four or five years that there is now a need for guidance on what molecular tests to order and how to use them to establish a diagnosis," said Brat, who is chair of pathology at Northwestern Medicine in Chicago.
Molecular testing is being performed for gliomas fairly comprehensively across the U.S., Europe, and much of South Asia and South America, but it's probably not being done to the extent that it should be, Brat said.
"The guideline gives a deeper dive and comprehensive analysis of the tests that really are needed for daily practice," he said.
Testing has implications for disease management and treatment even though targeted therapies are lacking for brain tumors, in contrast with other cancer types. For example, in glioblastoma, if MGMT is methylated, treatment with the chemotherapy drug temozolomide is more effective, Brat noted. And the presence of IDH mutations could be used to direct the timing of radiation therapy and/or chemotherapy.
Room to choose
CAP's recommendations provide guidance but are not intended to be comprehensive or prescriptive in terms of the types of tests that should be performed. For example, evaluating mutational status may be achieved with immunohistochemistry or gene sequencing.
"We are not attempting to tell pathologists and laboratories which ones they should do," Brat said. "There are going to be options and new tests that evolve."
CAP's recommendations were developed in cooperation with the American Society of Clinical Oncology, the Society for Neuro-Oncology, the American Association of Neuropathologists, and the Association for Molecular Pathology. The comment period for the recommendations will flag any major disagreements that could inform revisions, but multiple organizations provided input, and CAP believes the recommendations provide guidance on the best level of evidence in the literature, Brat said.