Hologic announced it has received clearance from the U.S. Food and Drug Administration (FDA) to market its new Genius Digital Diagnostic System digital cytology scanner platform plus artificial intelligence (AI) algorithm for cervical cancer screening in the U.S. The product has been available in Europe since November 2020.
Michael Quick, vice president of research and development and innovation in cytology and oncology at Hologic, told LabPulse.com that the new Genius Digital Diagnostic System's AI algorithm is a product of the company's ThinPrep imaging analysis software that has been in the market since 2003.
"Around 90% of the total Paps that are done in the U.S. today are done with the ThinPrep Imaging System, and the ThinPrep Pap test is the foundation," said Quick, who also serves as president of the Digital Pathology Association.
 Michael Quick, Hologic
Michael Quick, Hologic
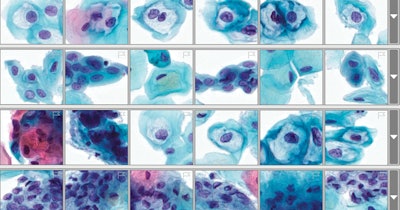
The Genius Digital Diagnostic System for cervical cancer screening uses what Hologic calls "volumetric scanning." Volumetric scanning is required for cytology, Quick explained. The scan captures the depth of the material in 3D from the glass microscope slide but compresses the volume of images into a single two-dimensional image. The device targets mid- to high-volume labs and can handle up to 400 slides. Its intended use replaces the microscope entirely, he said.
Acknowledging human papillomavirus (HPV) as a causative agent for cervical cancer, Hologic's new system is designed to complement molecular testing (Aptima) for HPV with cotesting for cervical cancer with the new digital cytology platform, according to Quick, who said the caveat would be if the user doesn't feel they can make an accurate diagnosis based on the images that are presented on the screen.
The new process and technology demonstrated an overall improvement in sensitivity without a corresponding decrease in specificity, Hologic said in a news release, noting there was a 28% reduction in false negatives of high-grade squamous intraepithelial and more severe lesions compared to microscopic review.
"A vast majority of women who have HPV will never develop cervical cancer," Quick said. "So it's identifying which women are at risk and also being able to screen out the women that you don't want to unnecessarily treat or refer. That's really the complementary nature of HPV and cytology and where Genius can fit in this paradigm of cotesting." Prior to Hologic's news release, Quick said the product was on track to receive de novo classification.
De novo classification provides a marketing pathway to classify novel medical devices for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device, according to the FDA's website. Devices that are classified into class I or class II through a de novo classification request may be marketed and used as predicates for future premarket notification [510(k)] submissions, when applicable.
According to Quick, the Genius Digital Diagnostic System for cervical cytology complements Hologic's existing Genius AI Detection software device used in digital breast tomosynthesis.



















