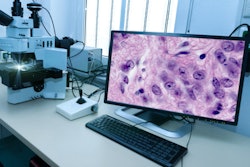
Leica Biosystems has received 510(k) clearance from the U.S Food and Drug Administration (FDA) for its Aperio GT 450 DX digital pathology system, in conjunction with Sectra's digital pathology software.
The Aperio GT 450 DX was previously sold in the clinical market under an enforcement discretion due to the COVID-19 pandemic. In a statement, Leica notes that the system is designed to deliver high-quality images with a rapid turnaround time of under 32 seconds per slide.
The FDA clearance includes Sectra's digital pathology solution when used with the Aperio GT 450 DX. The Linköping, Sweden-based Sectra noted in a statement that this is the first FDA clearance in digital pathology allowing the use of images in the DICOM file format for pathology diagnostics.
The two companies began collaborating on the integrated digital pathology system in 2019.