Self-assembling, multicellular biobots can traverse and fix damaged neural tissues in a simplified injury model, suggesting the tiny machines could have biomedical applications.
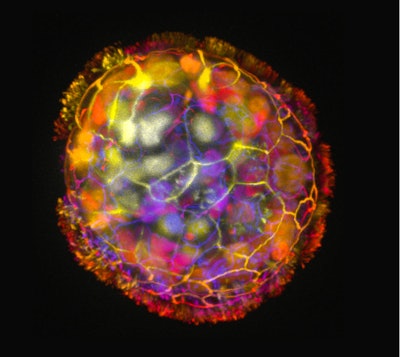
The discovery that the biobots can repair tissues came as a surprise. Writing in the journal Advanced Science, researchers at Tufts University and Harvard University’s Wyss Institute describe the creation of a type of biobot, which they call anthrobots, made from human cells. The anthrobots start as single lung cells and self-construct into larger structures when cultured in an extracellular matrix for two weeks.
Applied to live human neural monolayers in vitro, the anthrobots caused neurites to grow into gaps and join the opposite sides of the injury. The researchers have yet to determine what biochemical or biophysical properties of the anthrobots caused the healing but have generated evidence that the mini-machines are driving the repairs. Passive materials failed to repair the injury.
“It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage. We’re now looking at how the healing mechanism works, and asking what else these constructs can do,” Michael Levin, who holds positions at Tufts and Wyss, said in a statement.
In the paper, the researchers call the anthrobots’ repair ability “surprising,” explaining that it “could not have been guessed in advance from existing frameworks describing the uses of organoids and other bioengineered structures.”
The project shows the potential for emergent properties as human cells form into larger structures. The researchers took lung cells and, without introducing scaffolds or nanomaterials, grew multicellular anthrobots with a wide range of properties. The anthrobots displayed diverse motility patterns, with some moving in tight loops and others advancing in straight lines. The speed of movement varied as well.
Repairs happened despite the lack of immune components, migratory cells, inflammatory signaling, and other elements that support healing in complex in vivo systems. Studies of anthrobots in complex injury sites in vivo could reveal which of their properties and active processes are involved in healing tissues.