U.K. medical regulators have approved a “world-first” gene therapy that aims to cure two blood disorders: sickle cell disease (SCD) and transfusion-dependent β-thalassemia. They are the first regulatory body to approve the treatment.
The authorization brings hope to many patients with the painful conditions that affect quality of life and can cause life-threatening infections, although the costs of the therapy could impact access, say experts.
The treatment, Casgevy (exagamglogene autotemcel), is the first therapy to be licensed that uses the gene-editing tool CRISPR, the Medicines and Healthcare products Regulatory Agency (MHRA) announced.
Both SCD and β-thalassemia are hemoglobinopathies, inherited disorders caused by errors in the genes for hemoglobin, which is used by red blood cells to carry oxygen around the body.
The MHRA said that in clinical trials, Casgevy (named exa-cel in the U.S.) was found to restore healthy hemoglobin production in the majority of participants with SCD and transfusion-dependent β-thalassemia, relieving patients’ symptoms. The regulators’ authorization for patients age 12 and over was granted to Vertex Pharmaceuticals (Europe) and CRISPR Therapeutics.
The treatment “has the potential to significantly improve the quality of life for so many,” said John James, chief executive of the Sickle Cell Society.
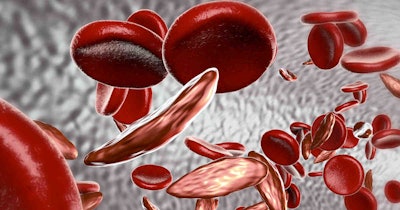
SCD is an inherited blood disorder that causes severe pain, organ damage, and shortened lifespan due to misshapen or “sickled” blood cells. It is particularly common in people with an African or Caribbean family background. About 15,000 people have the disorder in the U.K. People with transfusion-dependent β-thalassemia -- which primarily affects people of Mediterranean, South Asian, Southeast Asian, and Middle Eastern origin -- often need a blood transfusion every three to five weeks and injections and medicines throughout their lives.
“To date, a bone marrow transplant -- which must come from a closely matched donor and carries a risk of rejection -- has been the only permanent treatment option,” said Julian Beach, an interim executive director of the MHRA.
Casgevy is designed to work by editing the faulty gene in a patient’s bone marrow stem cells so that the body produces functioning hemoglobin. Stem cells are taken out of bone marrow, edited in a laboratory, and then infused back into the patient.
In the clinical trial for SCD, 28 out of 29 patients (97%) were free of severe pain for at least 12 months after treatment. In the trial for transfusion-dependent β-thalassemia, 39 out of 42 patients (93%) did not need a red blood cell transfusion for at least a year, while the remaining three patients had more than a 70% reduction in the need for red blood cell transfusions.
The MHRA said that no significant safety concerns were identified during the trials; the agency will continue to monitor Casgevy through real-world safety data and post-authorization safety studies.
“I hope this represents the first of many applications of this Nobel Prize winning technology to benefit eligible patients with serious diseases,” Samarth Kulkarni, PhD, chairman and CEO of CRISPR Therapeutics, said.
Experts said that the “landmark approval” opens the door for further applications of CRISPR therapies and potential cures for many genetic diseases.
“The challenge is that these therapies will be very expensive so a way of making these more accessible globally is key,” Dame Kay Davies, Dr. Lee’s Professor of Anatomy, University of Oxford, told the Science Media Center.
Vertex said it was already working closely with national health authorities to secure access for eligible patients “as quickly as possible.”
The gene therapy is also under review by the European Medicines Agency, the Saudi Food and Drug Authority, and the U.S. Food and Drug Administration.