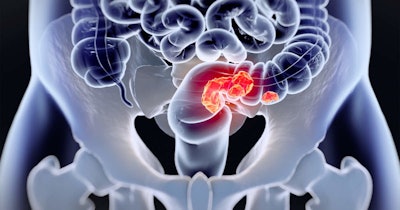
Liquid biopsy firm Universal DX and Quest Diagnostics have formed a strategic collaboration under which Quest will exclusively offer services for Universal DX’s Signal-C colorectal cancer (CRC) screening test in the U.S.
To support a submission to the U.S. Food and Drug Administration (FDA) for premarket approval for the Signal-C test, UDX will begin to enroll patients in a 15,000-patient study across more than 100 investigator sites to develop clinical evidence for the test. Testing for the study will be performed at Quest’s oncology center in Lewisville, TX. If the FDA approves the Signal-C test, Quest will have exclusive rights to provide clinical laboratory services in the U.S.
The Signal-C test uses next-generation sequencing to identify methylated DNA patterns and fragments in blood that are indicative of CRC tumors.
In a 1,000-patient study presented at Digestive Disease Week in May 2023, Signal-C demonstrated 93% sensitivity in detecting CRC and 54% sensitivity in detecting advanced adenomas at 92% specificity overall.
In a separate announcement, Universal DX announced that it has closed a series B financing round of approximately $70 million. Quest Diagnostics was one of the investors in the round.



















