Characterizing pancreatic cystic lesions with an investigational monoclonal antibody (mAb) Das-1 targeted test is simple, accurate, and potentially helpful for showing when surgery is unnecessary, according to a study published online in Gastroenterology on June 5.
In the blinded, retrospective analysis of 169 surgical samples from a range of tertiary referral centers, an enzyme-linked immunosorbent assay (ELISA) for the antibody Das-1 had an accuracy of 95% for identifying high-risk lesions and, therefore, which patients were most appropriate for surgery, reported a team led by Dr. Koushik Das, a Washington University gastroenterologist.
The researchers also found that the ELISA Das-1 test performed significantly better than criteria based on clinical guidelines for identifying high-risk lesions -- nonmucinous lesions are low-risk while mucinous types have varying degrees of malignancy.
"With a sensitivity of 88.2%, specificity of 99%, and overall accuracy of 94.7%, the biomarker was significantly more accurate than currently available guidelines (p < 0.001)," Das and colleagues concluded.
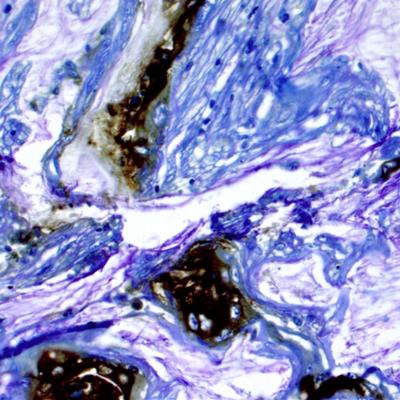
 Research led by the Washington University School of Medicine in St. Louis shows potential for a new way of identifying cysts in the pancreas that are likely to become cancerous. Above, brown areas are stained for a biomarker in tissue from a patient who developed pancreatic cancer from a cyst. Image courtesy of Dr. Koushik Das.
Research led by the Washington University School of Medicine in St. Louis shows potential for a new way of identifying cysts in the pancreas that are likely to become cancerous. Above, brown areas are stained for a biomarker in tissue from a patient who developed pancreatic cancer from a cyst. Image courtesy of Dr. Koushik Das.Tackling incidental findings
The researchers conducted the study to help address the conundrum of managing pancreatic cystic lesions, which are often detected in asymptomatic patients undergoing abdominal computed tomography (CT) and magnetic resonance imaging (MRI) scans. Resections in asymptomatic patients with cysts account for up to 20% of pancreatic resections in referral centers, and the vast majority of these are benign or indolent, Das et al noted. Various guidelines are available to help steer appropriate management, but their specificity and sensitivity are not high enough.
"Given the prevalence of asymptomatic cysts and possible morbidity associated with surgical interventions, there is an unmet need for molecular tools to risk-stratify lesions," they wrote.
Previous research has reported on the mAb Das-1 immunoassay's immunoreactivity against resected tissue in high-risk lesions; the results from a single-center study were presented at the American Gastroenterological Association (AGA) Digestive Disease Week meeting in 2017.
Das and two study co-authors -- Dr. Mari Mino-Kenudson, a Massachusetts General Hospital pathologist, and Dr. Kiron Das, PhD, a gastroenterologist at Rutgers Robert Wood Johnson Medical School -- hold a patent on the use of mAb Das-1 for detecting cancerous lesions of the pancreas.
It's still unclear exactly what antigen the mAb Das-1 test is reactive to. But the Das-1 antigen is expressed in normal fetal tissues in the gut and in adults with precancerous and cancerous conditions, Das et al noted.
The current study published in Gastroenterology represents a large sample size from four tertiary referral centers: 101 high-risk lesions were pathologically verified to have invasive carcinoma associated with a pancreatic cyst lesion, and there were also 68 low-risk lesions.
In this group, the Das-1 test performed better than the AGA guidelines, as well as the Fukuoka and Sendai guidelines, for identifying high-risk lesions such as invasive carcinomas, high-grade dysplasia, or intestinal-type intraductal papillary mucinous neoplasms with intermediate-grade dysplasia.
| Accuracy of classification for pancreatic cystic lesions | |
| Lesion classification tool | Accuracy |
| Das-1 assay | 94.7% |
| AGA guidelines | 73.7% |
| Fukuoka guidelines | 51.5% |
| Sendai guidelines | 46% |
Main pancreatic duct dilation of more than 5 mm, main pancreatic duct dilation of at least 1 cm, and jaundice were significantly associated with high-risk pancreatic cystic lesions, the investigators noted.
Das and colleagues also looked at results from carcinoembryonic antigen (CEA) tests, which are used to differentiate between mucinous and nonmucinous pancreatic cancer lesions, though there has been disagreement on what threshold to use, and accuracy has only been moderate. In a subset of 49 patients for whom preoperative cyst fluid was available, the sensitivity of CEA using a threshold of 192 ng/mL for identifying mucinous lesions was 50% and the specificity was 92.3%.
Prospective validation needed
A limitation of the study was that it was based on "retrospectively collected, prospectively banked, surgical specimens, from large, tertiary care centers, which introduces surgical selection, referral, and treatment access biases," the researchers wrote. The guidelines they used for comparison are intended for prospective use, not retroactive use on surgical samples. So it's unclear how the test would be integrated into a surveillance program -- the group is looking into validating the test on a prospective basis.
"Without a prospective cohort of patients followed, it is impossible to assess the malignant potential of a [pancreatic cystic lesion] within a patient's lifetime, however evaluation against a gold standard of blinded, pathology review is ultimately superior to surrogate end points and assumptions of indolence based on clinical/radiographic follow-up," Das et al wrote.
The authors also noted that multiple promising new methods are being positioned for a role in risk stratifying pancreatic cystic lesions, including a panel of next-generation sequencing targets with high accuracy for advanced neoplasia; however, these need to be validated in larger studies. Furthermore, they noted that ELISA is "simple, highly reproducible, and inexpensive," whereas technologies such as next-generation sequencing and mass spectrometry are more complex and require more skill.
"Indeed, the [noncommercialized] cost of performing the assay for an entire plate (40 samples) is $50-100," Das and colleagues wrote. "In addition, we found that the vast majority (81%) could be analyzed with as little as 125 [µL] of cyst fluid, and 94% could be completed with 500 [µL]. As the ELISA assay is further refined, likely smaller volumes of fluid will be required."