Castle Biosciences shared data from two studies on the performance of its DecisionDx-Melanoma test to predict the risk of metastasis and sentinel lymph node positivity in patients with cutaneous melanoma at the Society of Surgical Oncology 2024 (SSO 2024) annual meeting, held March 20-23 in Atlanta.
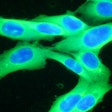
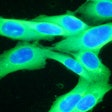
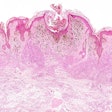
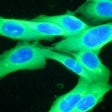
The DecisionDx-Melanoma test examines the gene expression profile or molecular signature of a tumor to assess a patient's risk of metastasis or sentinel lymph node (SLN) positivity.
Results from one study of 322 cutaneous melanoma patients who were being considered for an SLN biopsy to determine if the melanoma had spread to other lymph nodes showed that DecisionDx-Melanoma accurately predicted SLN positivity risk. No patients in this cohort with a DecisionDx-Melanoma predicted risk of SLN positivity under 5% had a positive SLN among all tumor stages studied. Furthermore, these patients remained SLN-negative and achieved 100% recurrence-free survival at three years. Castle noted in its statement that the predicted risk spared up to 25% of patients with T1-T2 tumors from having unnecessary invasive sentinel lymph node biopsies.
"The study that was orally presented at SSO 2024 demonstrates that patients who did avoid an [SLN biopsy] procedure had excellent outcomes to date. This demonstration is highly important as it showed that our test can help patients avoid an unnecessary procedure," said Derek Maetzold, president and CEO of Castle Biosciences.
The other study, with a pooled cohort of 979 patients with thin (T1) tumors, demonstrated that the DecisionDx-Melanoma can also identify patients at higher risk for SLN positivity and recurrence who should be considered for more intensive management, including SLN biopsies and increased frequency of follow-up and imaging surveillance, in order to improve patient outcomes.