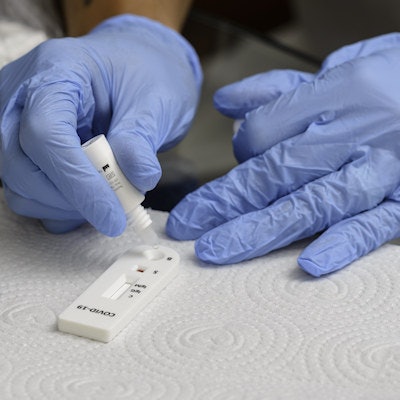
The U.S. Food and Drug Administration (FDA) has granted an emergency use authorization (EUA) for Visby Medical's COVID-19 reverse transcription polymerase chain reaction test for pooled samples.
The authorization expands the company's first EUA for single-patient-sample testing to pooled testing of up to five samples at once for SARS-CoV-2, Visby said.



















