A microfluidic device being developed at the Massachusetts Institute of Technology (MIT) could enable sepsis to be diagnosed more quickly and with a smaller sample of blood, according to a paper being presented by MIT researchers this week at the Engineering in Medicine and Biology Conference in Berlin.
Sepsis occurs when the body's immune response to an infection spurs a chain reaction of inflammation. Sepsis is a leading cause of death in hospitals in the U.S., resulting in nearly 250,000 patient deaths a year.
Existing techniques for diagnosing sepsis include monitoring vital signs, medical imaging scans, and lab and blood tests. Protein biomarkers such as interleukin-6 (IL-6) are emerging as possible early indicators of sepsis, with rising IL-6 levels indicating that sepsis could be imminent.
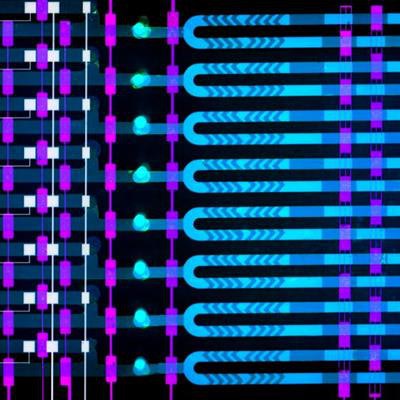
 The microfluidics device developed at MIT includes eight channels measuring 1 micron each, through which a blood sample is mixed with microbeads laced with an antibody to help detect rising levels of IL-6, a biomarker for sepsis. The device delivers results in less than 25 minutes. Image courtesy of Felice Frankel.
The microfluidics device developed at MIT includes eight channels measuring 1 micron each, through which a blood sample is mixed with microbeads laced with an antibody to help detect rising levels of IL-6, a biomarker for sepsis. The device delivers results in less than 25 minutes. Image courtesy of Felice Frankel.Traditional assays have difficulty detecting IL-6 at low concentrations, and the machines involved are often bulky and expensive. What's more, they require about a milliliter of blood and take hours to deliver results. Newer point-of-care machines are promising, but these can also be expensive and detect only a small number of proteins.
The MIT research team has developed technology that can detect rising IL-6 levels using less than a finger prick of blood and deliver results in about 25 minutes. The paper includes first author Dan Wu, a doctoral student in MIT's department of mechanical engineering, and Joel Voldman, PhD, an associate professor of electrical engineering and computer science.
The MIT team essentially developed a miniaturized version of a magnetic bead-based assay and adapted it to an automated microfluidics device that is several square centimeters in size. The device has eight micron-sized channels for transporting fluid.
A blood sample is loaded into the device with a pipette, along with microbeads laced with an antibody that attracts IL-6 proteins, which bind to the antibodies in about 10 minutes. In a separate channel, only beads that contain the biomarker attach to an electrode; when voltage is run through the electrode, it produces an electrical signal for each bead with the biomarker, using a technique called amperometry. The device then counts the signals and calculates the concentration of IL-6.
The device operates with about 5 µL of blood -- about one-fourth the volume drawn from a finger prick and far less than the 100 µL used in lab-based assays for protein biomarker detection. It is capable of detecting concentrations of IL-6 as low as 16 pg/mL, which is below the concentration that signals sepsis, according to the researchers.
Wu and colleagues hope to continue their work by adapting the device to detect a panel of biomarkers for sepsis in addition to IL-6, including interleukin-8, C-reactive protein, and procalcitonin. More biomarkers could be detected by simply adding more microfluidic channels beyond the eight currently used, according to the group.