Some insurance companies restrict noninvasive prenatal testing to women who are older than 35 and have high-risk pregnancies. But testing also makes sense in younger pregnant women and can help decrease the use of amniocentesis and other invasive tests, according to a study presented on October 4.
In the real-world study of claims data, Harvard Pilgrim Health Care and Illumina worked together to dramatically expand the use of prenatal blood testing to younger pregnant women while reducing the number of invasive tests, such as amniocentesis.
The investigators evaluated the effects of a program started in March 2017 to expand access to noninvasive prenatal testing (NIPT) with a single blood draw among pregnant women younger than 35 who were insured by Harvard Pilgrim Health Care. NIPT screens for a range of fetal chromosomal disorders, including trisomy 13 (Patau syndrome), trisomy 18 (Edwards syndrome), and trisomy 21 (Down syndrome).
Adjusting for differences in the number of unique pregnancies, the annual number of noninvasive prenatal tests rose by 42% in 2018-2019, compared with the period before the risk-sharing agreement took effect. The investigators reported a 15% decline in the number of orders for invasive tests -- chorionic villus sampling and amniocentesis -- during that period.
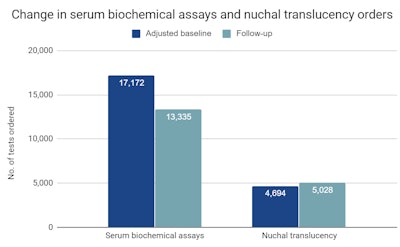
 Adjusted baseline = March 1, 2016, to February 28, 2017; follow-up = March 1, 2018, to February 28, 2019. Courtesy of University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences.
Adjusted baseline = March 1, 2016, to February 28, 2017; follow-up = March 1, 2018, to February 28, 2019. Courtesy of University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences.The study is based on an analysis of claims data including Current Procedural Terminology (CPT) codes for pregnancy-related diagnostics and procedures performed on women younger than 35 before (2016-2017) and after (2018-2019) NIPT access was expanded at Harvard Pilgrim Health Care.
The research was conducted by reimbursement intelligence company Real Endpoints and Illumina, using the insurance claims data provided by Harvard Pilgrim, and the data were analyzed by researchers at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The results were shared in a poster presentation by co-author R. Brett McQueen, PhD, a health economist at the University of Colorado, on October 4 at the American College of Obstetricians and Gynecologists Districts V, VIII & IX annual meeting in Maui, Hawaii.
Noninvasive prenatal testing is more accurate than traditional serum biochemical and nuchal translucency screening tests for fetal anomalies and, consequently, is less likely to result in invasive follow-up tests, McQueen and colleagues at Illumina, Real Endpoints, and Harvard Pilgrim wrote in their poster presentation. But insurance coverage sometimes restricts access to NIPT for women older than 35 with high-risk pregnancies, they noted in a statement.
A full analysis of the data, including effects on cost and outcomes, is expected in 2020.