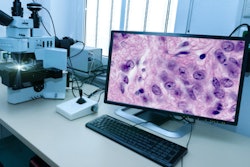
Numares has submitted a 501(k) application to the U.S. Food and Drug Administration (FDA) for its Axinon nuclear magnetic resonance (NMR) IVD platform for metabolomics-based and artificial intelligence (AI)-driven diagnostics.
 The Axinon NMR system. Image courtesy of Numares.
The Axinon NMR system. Image courtesy of Numares.Axinon aims to identify early kidney rejection in post-transplant surveillance, assess kidney function by improved determination of glomerular filtration rate, and ascertain risk for cardiovascular disease. If cleared, Axinon would become the first NMR-based clinical laboratory system to use AI-evaluated metabolic data, according to Numares.