Karolinska Institutet researchers and their colleagues found that a protein called GFAP is a possible biomarker for very early stages of an inherited form of Alzheimer’s disease. The study, published January 11 in the journal Brain, could potentially lead to earlier detection of this serious disease.
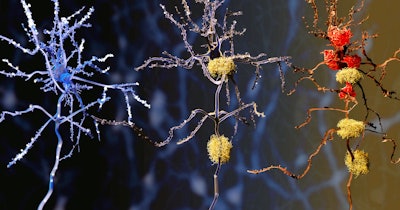
Alzheimer’s disease causes about two-thirds of all dementia cases. Nerve cells in the brain degenerate as a result of the abnormal accumulation of proteins, including amyloid-beta peptides and neurofibrillary tau tangles. The increasing neuron damage results in cognitive dysfunction, including impaired memory and speech. Some biological changes in the brain may occur 20 to 25 years before symptoms become evident.
The researchers hypothesized that emerging blood plasma biomarkers of Alzheimer's disease might be noninvasive tools with which to trace early Alzheimer's disease-related abnormalities. They studied blood biomarkers for very early pathological changes in a rare and inherited form of Alzheimer’s disease called autosomal dominant Alzheimer's disease that accounts for less than 1% of all cases. Individuals with a parent who developed this mutation-caused Alzheimer’s disease have a 50% risk of developing the disease as well.
Seeking to explore the timing and performance of plasma biomarkers in mutation carriers compared to non-carriers, the researchers analyzed 164 blood plasma samples. These samples were collected between 1994 and 2018 from 33 mutation carriers and 42 relatives without the inherited predisposition.
The results revealed significant changes in several blood protein concentrations in the mutation carriers, measured with a single-molecule array method. Subsequent longitudinal analyses confirmed that plasma P-tau181, neurofilament light protein (NfL), and glial fibrillary acidic protein (GFAP) concentrations were higher in mutation carriers compared to non-carriers. Changes were observed in the presymptomatic phase, detectable first as an increase in GFAP approximately 10 years before estimated symptom onset, followed by increased levels of P-tau181 and NfL closer to expected symptom onset.
“Our results suggest that GFAP, a presumed biomarker for activated immune cells in the brain, reflects changes in the brain due to Alzheimer’s disease that occur before the accumulation of tau protein and measurable neuronal damage,” said Karolinska Institutet doctoral student and first author Charlotte Johansson in a statement. “In the future, it could be used as a noninvasive biomarker for the early activation of immune cells such as astrocytes in the central nervous system, which can be valuable to the development of new drugs and to the diagnostics of cognitive diseases.”