Inova Diagnostics has secured emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its Quanta Flash SARS-CoV-2 immunoglobulin G (IgG) assay.
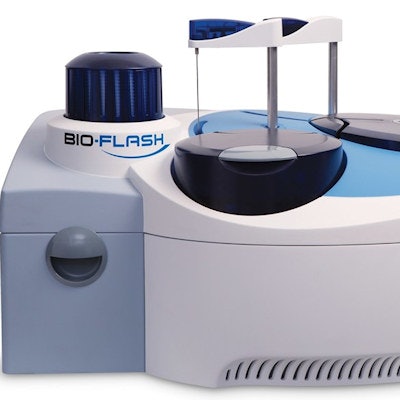
The assay is for use on the company's Bio-Flash random access chemiluminescent analyzer, which has a processing capacity of up to 60 samples per hour, according to the firm. It has shown 100% specificity for identifying SARS-CoV-2 in patients "with confounding conditions" and 100% sensitivity for COVID-19 in patients 15 days after a positive reverse transcription polymerase chain reaction (RT-PCR) result, Inova said.
 Inova Diagnostics' Quanta Flash rapid response chemiluminescent assay runs on the Bio-Flash lab analyzer. Image courtesy of Inova Diagnostics.
Inova Diagnostics' Quanta Flash rapid response chemiluminescent assay runs on the Bio-Flash lab analyzer. Image courtesy of Inova Diagnostics.


















