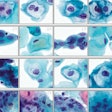
BioReference Health on Thursday announced that it is now offering the Roche Diagnostics CINtec Plus Cytology test, the only U.S. Food and Drug Administration (FDA)-approved dual-stain triage test for patients who have a high-risk human papillomavirus (HPV) result.
Healthcare providers can order the test through BioReference and its specialty division, GenPath Women’s Health.
The test uses dual biomarker technology to simultaneously detect p16 and Ki-67 in women with HPV-positive results. Co-expression of these two biomarkers within the same cell is a strong indicator that an HPV infection is undergoing oncogenic transformation, according to Roche.
BioReference will offer the test to women from ages 30 to 65 as a reflex when the cytology result is negative for intraepithelial lesion or malignancy (NILM) and the high-risk HPV result is positive using Roche’s Cobas HPV test.
The dual-stain biomarker test allows a treating healthcare provider to more accurately and quickly assess the risk for cervical pre-cancer in patients, BioReference said, adding that the test can reduce the number and frequency of follow-up visits.