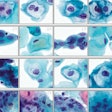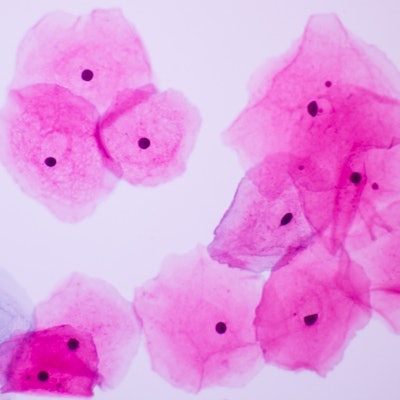
Agilent Technologies has secured the CE-IVD Mark in Europe for its companion diagnostic test for identifying cervical cancer patients eligible for treatment with Merck's Keytruda (pembrolizumab) immunotherapy.
PD-L1 is an antibody expressed by cervical cancer tumors and is a key biomarker for predicting response to anti-PD-L1 therapies like Keytruda, according to Agilent. The company's PD-L1 IHC 22C3 pharmDx companion diagnostic identifies cervical cancer patients with a PD-L1 combined positive score of one or more, which indicates that they could benefit from treatment with Keytruda, the company said.