The U.S. Food and Drug Administration has cleared Scopio Labs' X100 with full field peripheral blood smear (full field PBS) product for use with in vitro hematology diagnosis.
The full field PBS system uses computational photography imaging and artificial intelligence to capture digital scans with a full field view of the monolayer and feathered edge at 100 times the oil immersion resolution level, the firm said. The system also uses monolayer identification to support long and short smears and automates the analysis process by preclassifying 200 white blood cells, providing platelet pre-estimate, and enabling red blood cell morphology evaluation.
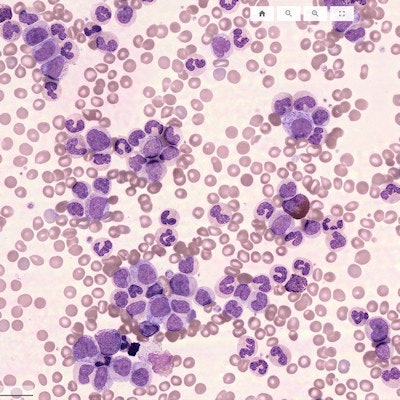
 Scopio’s full field PBS product. Image courtesy of Scopio.
Scopio’s full field PBS product. Image courtesy of Scopio.The full field PBS product includes a three-slide tray and a decision support system for that preclassification.
Scopio Labs said it is setting its sights on transforming all hematology applications, including bone marrow aspirates and body fluids.