NowDiagnostics has received an emergency use authorization (EUA) from the U.S. Food and Drug Administration for AdexusDx, a point-of-care test to detect antibodies to SARS-CoV-2, the virus that causes COVID-19.
AdexusDx is designed for use in moderate-complex settings and at the point of care, according to NowDiagnostics. The company said the test can be used in a variety of CLIA-waived healthcare settings that include pharmacies, clinics, and hospital emergency rooms.
The company is also conducting clinical studies to make AdexusDx available on an over-the-counter basis for detection of SARS-CoV-2 antibodies. The test uses a 40-µL drop from a fingerstick or venous whole blood, serum, or plasma to identify individuals with SARS-CoV-2 antibodies. NowDiagnostics believes the test's simplicity and portability mean that it could be used in a wide variety of environments.
Development of the test was funded in part by the U.S. Biomedical Advanced Research and Development Authority (BARDA).
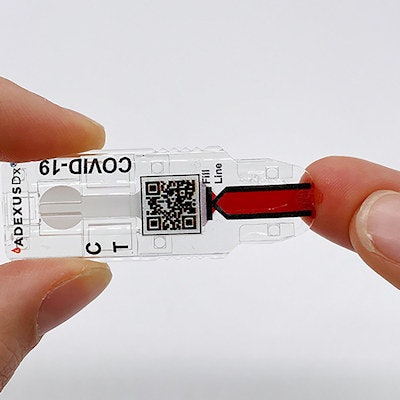
 The AdexusDx COVID-19 test is a rapid serology, self-contained assay that measures the presence of SARS-CoV-2 antibodies with no buffers, reagents, or additional equipment. Image courtesy of NowDiagnostics.
The AdexusDx COVID-19 test is a rapid serology, self-contained assay that measures the presence of SARS-CoV-2 antibodies with no buffers, reagents, or additional equipment. Image courtesy of NowDiagnostics.

















